Clean Corrosion Off Electrical Contacts
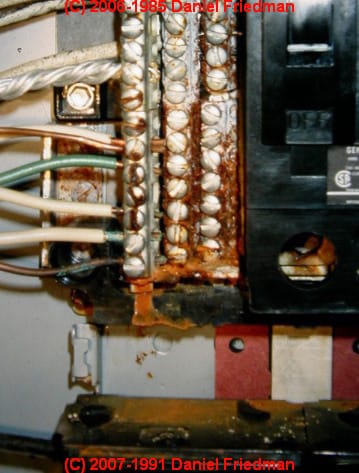
When electrical wires are left alone too long in the elements the metal in the wires can corrode.
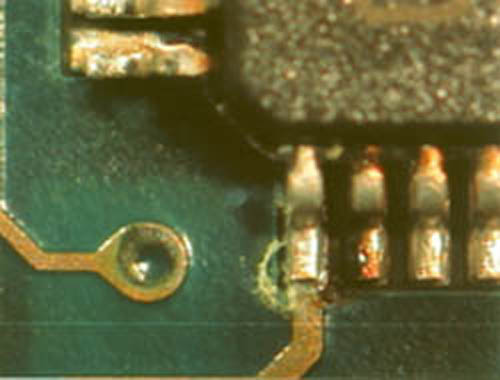
Clean corrosion off electrical contacts. Then apply the paste to the surface of your item and let it sit for 2 hours. Oxidation of metal parts because of humidity and old leaking batteries is a common cause of corrosion. The corrosion can impede the flow of electricity and cause failure to the device that is being powered. Most often a basic cleaning on the male and female parts can remedy the dirty situation.
The acidity of the vinegar will eat away at the rust making it easier to remove. Electrical connections especially those exposed to the outdoor elements can be prone to corrosion. You should be able to find the offending battery fairly easily because it will be corroded as well. Corrosion is usually caused by acid leaking from a battery near the circuit board.
Copper contacts can also become dirty with black scuff marks if the contacts have something inserted and removed on a regular basis. First mix together lemon juice and salt to form a paste. Alkaline batteries leak not acid but a chemical that registers as a base on the ph scale. Use as many swabs as you need to get them clean.
Step 3 use an eraser. Generally the male parts. You can also use lemon juice and salt to remove rust and corrosion. After 2 hours scrub the paste off with a soft bristled brush.
This is especially the case with cartridge video games. Continue to scrub the copper contacts until the head of the cotton swab is black. Corrosion on electronic contacts can dramatically impact the performance of a device which may become inoperable if the corrosion clogs the power contacts to the point the electrical circuit is broken. If connected to a battery the subsequent corrosion can take the form of acid leakage.
Remove the battery with rubber gloves clean away any corrosion it left in the battery socket and insert the replacement battery. You can save those expensive electronic gadgets and. Electrical wires are usually made from copper and as such prone to oxidation.